Choosing parental varieties
Selecting parental varieties for crossing is usually straightforward. Various factors affect the suitability of any given pair of varieties and the likelihood of a successful outcome:-
- Phenotype for the desired trait(s)
- Degree of common ancestry
- Pollen incompatibility
- Flowering dates
- Self-fertility of the female parent
- Triploid/diploid status
The approach taken in most breeding programmes is to choose parents with superior phenotypes for the key traits of interest and to select the best of the progeny, sometimes using them as parents in further crosses. This quote from The Midwest Apple Improvement Association News Letter, 1999, No.1 is instructive here:
‘When we asked Dr. Jules Janick whether it was a better breeding strategy to repeat past successful crosses to generate still larger numbers of progeny to choose from or use as parents the best selections out of the existing progeny, he said always cross the best available with the best available moving forward. However this may mean making frequent use of a 'killer" parent from the past like Golden Delicious.’
This approach is exemplified by the historical popularity of Cox’s Orange Pippin as a parent in UK breeding programmes during the twentieth century.
Janick et al. (1996) highlight the fact that if the breeder’s aim is to introduce a character controlled by a major gene or genes, the choice of at least one parent is limited to cultivars carrying these genes. However, most of the important traits are controlled by many genes (polygenic), meaning that there will be a considerable range of variation in parental phenotype available for use. According to Janick et al. (1996), the breeding behaviour of highly heterogeneous parental cultivars with respect to measurable characters having a low level of genetic dominance is often predictable. The mean value for the character in the progenies falls with reasonable consistence around the mean of the two parents. Consequently they recommend combining parents that between them have all the chosen characters present at as near an optimal expression as possible. In other words, for a given trait cross ‘good’ with ‘good’. However, these authors add that it may be more realistic to choose parents with complementary characteristics in order to select progeny that have the desirable attributes of each parent.
Degree of common ancestry
Historically, the repeated use of ‘good’ x ‘good’ parents in apple breeding has led to a significant degree of genetic narrowing, because the same varieties have been used as parents again and again, akin to inbreeding. The consequences of this are discussed by Bannier (2011) in an article on ‘inbreeding in modern apple cultivation’, available in translation on Nigel Deacon’s website. The author traces back the lineage of many modern cultivars to include six common varieties. Out of 500 varieties assessed, all developed since 1920, the lineage of 274 varieties includes one of the ‘six ancestor varieties’ twice or more in the family tree back to great-grandparent. The six ancestor varieties are Golden Delicious (in 255 varieties), McIntosh (174 varieties), Jonathan (154), Cox’s Orange Pippin (150), Red Delicious (90) and James Grieve (75 varieties). The parentage of the 500 selected varieties is provided in the Appendix to this article. The author argues that the consequences, in terms of genetic narrowing and loss of ‘vitality’, are undesirable and suggests using parents without the six ancestor varieties in their lineage.
We try to follow Bannier’s advice in our breeding programme. Many of the crosses we make are between parents without the six ancestor varieties in their lineage. However, others we make don’t satisfy this criterion. This is because many cultivars with the six ancestor varieties in their lineage are superior in terms of the specific traits we are interested in.
Pollen incompatability
Most apple varieties are partly or fully self-incompatible (self-sterile), due to the failure of the pollen tube, arising from their own pollen, to grow down the style. When the incompatibility is only partial, the variety is often referred to as ‘self-fertile’ (e.g. Lord Lambourne), meaning that it can be fertilised by its own pollen. However, even self-fertile varieties set more fruit, with higher seed content, when they are pollinated by an unrelated variety.
Incompatibility is genetically controlled by the so-called 'S locus alleles'. A good review of the genetics is given by Hegedűs (2006). According to this author, ‘fruit set behaviour is controlled gametophytically by a locus, comprising a multigene complex, with an S-RNase gene expressed in the pistils and an S-haplotype-specific F-box gene expressed in the pollen tubes. The process of self/non-self recognition and the consequent acceptance or rejection takes place between the protein products of these genes. Cultivars sharing common S-genotypes are mutually self-incompatible, hence crossing them will not result in progeny.’
How does this work in practice? At least 29 different versions, or alleles, of the responsible gene complex have been identified, referred to as S1-S29. Each cultivar/variety has a pair of S-alleles, inheriting one allele from each of its parents. In most cases the S-locus is heterozygous; in other words the two alleles are different. For example, Cox’s Orange has S5 & S9, whilst Golden Delicious has S2 & S3. However, a few varieties are homozygous; in other words both alleles are the same. For example, Irish Peach has S1 & S1. The versions of the S alleles in many, but not all, cultivars and varieties have been identified and listed (e.g. Hegedűs, 2006).
Varieties are ‘fully incompatible’ if they share the same pair of S-alleles. This applies, for example, to Lord Lambourne and Falstaff, both of which have S2 & S5 alleles. Consequently, pollen from Falstaff will not fertilise Lord Lambourne and vice versa. Varieties are regarded as ‘semi-compatible’ if they share one of their S-alleles; for example Discovery (S1 & S24) and Ellison's Orange (S1 & S5). In these cases only 50% of the pollen produced will be capable of germination and growth down the style to fertilise the ovule. Nevertheless, semi-compatible varieties will fertilise each other, and they can usually be crossed successfully. However, the success rates are unlikely to be as high as achieved in crosses between ‘fully compatible’ varieties, those with no S-alleles in common, such as Spartan (S9 & S10) and Discovery (S1 & S24). Triploid cultivars behave in a similar way to diploids (Janick et al.,1996), varying in the extent of self-incompatibility. They produce a better fruit set when pollinated with diploids, but vary considerably when crossed with other triploid varieties.
Hegedűs (2006) states that the widespread use of ‘Golden Delicious’, ‘Delicious’, ‘Jonathan’, ‘McIntosh’ and ‘Cox's Orange Pippin’ in breeding programs has resulted in the accumulation of their S-alleles (S2, S3, S5, S7, S9, S10 and S28) in cultivars and varieties. This concentration of S-alleles increases the chances of incompatibility in crossing programmes relying on these elite varieties as parents. It also resonates with Bannier’s (2011) concern regarding the genetic narrowing of the apple breeding base.
We avoid using ‘fully incompatible’ varieties as parents in our breeding programme mainly because they are a waste of effort. However, we often cross semi-compatible varieties, with varying success rates. For example, two out of three Braeburn (S9 & S24) X Discovery (S10 & S24) crosses failed due to no fruit set, whilst two out of two Honeycrisp (S2 & S24) X Discovery (S10 & S24) were successful. On the other hand, selecting fully compatible varieties as parents does not guarantee success. For example, three out of four Beauty of Bath (S1 & S4) X Lord Lambourne (S2 & S5) crosses failed.
Self-fertile varieties
The majority of varieties are not self-compatible (self-fertile) under normal circumstances. However, the list of varieties classified as self-fertile varies between authorities according to the inclusion of those observed to be rarely, or partially, self-fertile (e.g. James Grieve, Lord Lambourne). We try to avoid partially or fully self-fertile varieties as female parents, because pollen from adjacent flowers might be wind-transferred onto the emasculated flowers prepared for the cross, even though these are ‘bagged’ to prevent insect pollination.
Flowering dates

We mainly use parental varieties with overlapping flowering dates for crossing, particularly if we want to hand pollinate with freshly collected pollen from the male parent. This doesn’t often constrain our choice of parents because there is usually a significant overlap in flowering dates (image opposite). Commercial growers in The Netherlands are advised that that the average flowering ranges of any two varieties should overlap by a minimum of six days (Kemp and Wertheim, 1992). However, if this is inconvenient, the flowers from early flowering varieties to be used as male parents with later flowering female parents, can be collected and stored in open topped plastic vials for a few days or weeks until the pollen dehisces from their anthers; then used to pollinate female parents. Additionally, pollen can also be collected this way and stored dry over much longer periods for later use. It remains viable for more than a year when stored in loosely stoppered vials in a desiccator containing Calcium Chloride at -15°C (Janick et al., 1996). Stored in a desiccator at 3°C, it remains viable for up to 4 years (AHDB, 2010).
Flowering dates also vary from year to year, depending on the weather. Ten year average flowering dates for over 1300 varieties are listed by MAFF (1973) and show that full flowering differs by 39 days between the earliest and the latest flowering varieties. An average flowering period of 16 days has been quoted, based on Danish research (Grausland, 1996) on 11 apple varieties using 10 year averages.
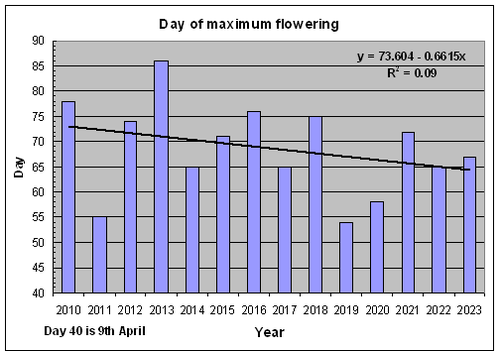
Our observations suggest that over recent years flowering may generally be occurring a little earlier in the spring. For example, the bar-chart opposite shows the date (Day 40 is 9th April) of peak flowering averaged across a sample of 22 varieties (including the 18 shown in the previous graph), measured in successive years between 2010 and 2023. Visually, the data suggests an overall trend, subject to wide year to year variation, towards earlier peak flowering dates. This is also indicated by the slope of the straight line given by linear regression of peak flowering day on year (2010 = 1, 2013 = 14). However, the correlation is very weak.
References
AHDB 2010. Membership of the East Malling rootstock club, Project TF182. Agriculture and Horticulture Development Board 2010 UK.
Bannier HJ. 2011. Inbreeding in Modern Apple Cultivation. Erwerbs-Obstbau 52 (3): 85-110. Version Translated by Reinhard Schomberg-Klee and Nigel Deacon at link below
Grauslund J. 1996. Flowering dates of pome and stone fruit cultivars – 10 years results. Acta Horticulturae, 423: 31-37.
Janick J, Cummins JN, Brown SK and Hemmat M. 1996. Apples. In: Janick J, Moore JN, (eds), Fruit Breeding, Volume I: Tree and Tropical Fruits. John Wiley & Sons, Inc. 1-77.
Hegedűs, A. 2006. Review of the self-incompatibility in apple (Malus × domestica Borkh., syn.: Malus pumila Mill.) International Journal of Horticultural Science 12 (2): 31–36.
Kemp H. and Wertheim SJ. 1992. Bestuiving, 18e Rassenlijst voor ruitgewassen, 14-20.
MAFF. 1973. Flowering Periods of Tree and Bush Fruits. Ministry of Agriculture, Fisheries and Food, Technical Bulletin 26. HMSO, London. 76p.
MAIA, 1999. Midwest Apple Improvement Association News Letter 1999, No. 1.